Electronic and crystal structures of 1,2,3-triazole-fused p-benzoquinone derivatives†
Abstract
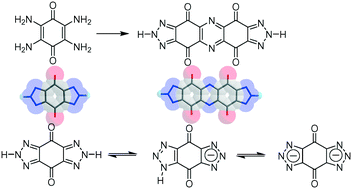
The preparation and crystal structure analyses of 1,2,3-triazole fused p-benzoquinone derivatives were performed conventionally from electronically active tetraamino-p-benzoquinone (1). The redox behaviour and crystal structure of 1 were compared with those of chemically stable strong electron donor tetrakis(dimethylamino)-p-benzoquinone (3). The reaction of 1 with NaNO2 generated a diazonium salt, which reacted further to give residual amino groups of 1, forming bis(1,2,3-triazole)-p-benzoquinone (2) in high yield. Along with intramolecularly cyclized compound 2, novel π-extended dimeric product bis(1,2,3-triazole)-phenazinetetraone (4) was also generated in this reaction and isolated in charge-transfer complexes with perylene and coronene. The molecular structures of neutral 2, monoanionic 2−, and dianionic 22− were confirmed by single crystal X-ray structural analyses, where the acidic protons in 2 existed in the 2-position of the 1,2,3-triazole unit, whereas one proton in 2− was localized at the 1-position of the 1,2,3-triazole unit. The electron accepting ability of bis(1,2,3-triazole)-p-benzoquinone derivatives decreased in the order 2 > 2− > 22− due to electrostatic repulsive interactions in the anion radical and dianion state. Hydrogen-bonded new electron acceptor 4 was surrounded by four H2O molecules in the crystalline state, which formed an alternating donor–acceptor π-stacking interaction.

 Please wait while we load your content...
Please wait while we load your content...