Solid state supramolecular structure of diketopyrrolopyrrole chromophores: correlating stacking geometry with visible light absorption†
Abstract
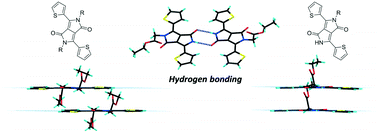
Mono- and di-alkylated 1,4-diketo-3,6-dithiophenylpyrrolo[3-4-c]pyrrole derivatives (TDPPs) have been synthesised and their solid state packing and absorption properties have been correlated. In this library of compounds the bulkier substituents distort the geometry of the chromophores and shift the lowest energy absorption band as a consequence of reduced π–π stacking and inter-chromophore overlap. Longitudinal displacement of the conjugated core is affected by donor–acceptor intermolecular interactions and twisting of the thiophene ring out of the plane of the DPP core, whereas lateral displacement was correlated to distortion of the NLactam–C(R) bond out of the plane of the DPP core. The di-substituted TDPP with hexyl units exhibit high molecular planarity, strong close packing of the conjugated core and significant red shift of the maximum of absorption in the solid, whereas the mono-substituted compounds with hexyl and ethyl acetate units are the least distorted of the series because of strong intermolecular hydrogen bonding that increases the molecular overlap and planarity of the chromophores. Therefore the family of mono-substituted TDPPs and more specifically the ones with ethyl acetate substituents show good potential for modulating the molecular geometry and optimizing the charge transport in materials for organic electronic applications.


 Please wait while we load your content...
Please wait while we load your content...