Cu–Ln compounds based on nitronyl nitroxide radicals: synthesis, structure, and magnetic and fluorescence properties†
Abstract
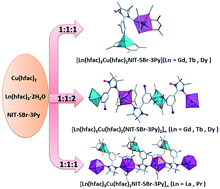
Three series of nitronyl nitroxide radical 3d–4f compounds, namely, [Ln(hfac)3Cu(hfac)2NIT-5Br-3Py]n (LnIII = La (1), Pr (2)), [Ln(hfac)3Cu(hfac)2NIT-5Br-3Py] (LnIII = Gd (3), Tb (4), Dy (5)) and [Ln(hfac)3Cu(hfac)2(NIT-5Br-3Py)2]n (LnIII = Gd (6), Tb (7), Dy (8); NIT-5Br-3Py = 2-(5-bromo-3-pyridyl)-4,4,5,5-tetramethyl-imidazoline-1-oxyl-3-oxide; hfac = hexafluoroacetylacetonate) have been successfully obtained. Single crystal X-ray crystallographic analysis reveals that complexes 1 and 2 consist of 1D chains built up by Ln(hfac)3 units bridged by the radical ligands through their NO moieties and the additional Cu ions are coordinated to the nitrogen atoms of the radicals. Complexes 3–5 are rare hetero-binuclear Cu–Ln compounds bridged by the nitronyl nitroxide radicals while complexes 6–8 possess one-dimensional chain structures with a repeating [Cu–Rad–Ln–Rad] sequence. DC magnetic studies show ferromagnetic interaction between the LnIII ion and the coordinated NO group in compounds 3–8. Tb–Cu derivatives (complexes 4 and 7) display frequency dependent ac magnetic susceptibilities, indicating slow magnetic relaxation behavior. The room-temperature photoluminescence spectra of complexes 4 and 7 exhibit strong characteristic emissions in the visible region.

 Please wait while we load your content...
Please wait while we load your content...