Lattice interactions of terpyridines and their derivatives – free terpyridines and their protonated forms†
Abstract
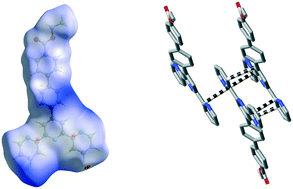
Consideration of the crystal structures of four new 2,2′;6′,2′′-terpyridine derivatives in terms of the weak lattice interactions which can be discerned through calculation of their Hirshfeld surfaces shows that despite the common feature of stacking of the near-planar aromatic entities, specific interactions beyond dispersion within the stacked arrays are relatively uncommon. This is in accord with what is seen in the same analysis of the structures of known analogues, which provides context for the present work. That this is not simply a consequence of the transoid array of the N-donors in these free ligands is seen from extension of the Hirshfeld surface analysis to the structures of protonated species where cisoid arrays are seen.

 Please wait while we load your content...
Please wait while we load your content...