Microphase separation upon crystallization of small amphiphilic molecules: ‘low’ temperature form II of sodium benzoate (E 211)†
Abstract
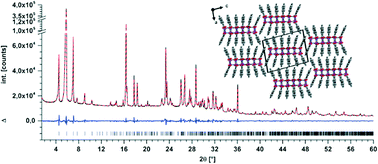
A so far unknown low temperature polymorph (form II crystallized at 350 °C) of the common food preservative sodium benzoate (NaBz; E 211) could be crystallized by annealing (7 days) of the poorly crystalline technical material. Its crystal structure was solved by indexing a tiny single crystal by electron diffraction followed by structure solution and refinement applying powder X-ray diffraction (PXRD). The new polymorph shows many structural similarities to form I (crystallized at 420 °C). Both form I and II are the result of a microphase separation: the structures consist of pseudo-hexagonal packings of rod-shaped micelles where the core consists of sodium cations being coordinated by the carboxylic groups, while the phenyl moieties of benzoate point outwards rendering the micelle surface hydrophobic. The structures differ by the degree of elliptical distortion of the rods which is more pronounced for the new form II due to incorporation of an additional Na-benzoate moiety into the circumference of the rods.

 Please wait while we load your content...
Please wait while we load your content...