Intermolecular interactions and chiral crystallization effects in (1,5,3-dithiazepan-3-yl)-alkanoic acids†
Abstract
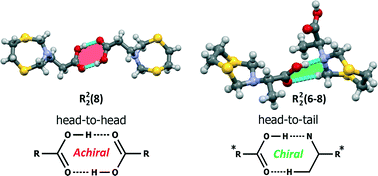
Crystals of (1,5,3-dithiazepan-3-yl)-alkanoic acids with achiral and chiral amino acid moieties have been obtained, and their structures were studied using a single crystal X-ray technique. Simple crystal systems, namely monoclinic, triclinic, and orthorhombic, were revealed in a series of the studied compounds. The reference 1,5,3-dithiazepan-3-ol forms head-to-head cyclic dimeric associates R22(6) via strong O–H⋯N intermolecular hydrogen bonds. The achiral 1,5,3-dithiazepanes form head-to-head cyclic dimers R22(8) between two carboxylic groups, whereas the co-crystals involve solvent molecules to constitute dimeric pairs R33(11) and R22(8) through O⋯H and N⋯H hydrogen bonds. These dimers further contribute to the aggregation of layers and stacks due to diverse H⋯H and S⋯H contacts. The chiral dithiazepanes form head-to-tail R12(6), R22(7), and R22(8) cyclic dimers between the carboxylic group of one molecule and the dithiazepane moiety of the other one through H⋯H, S⋯H, and O⋯H intermolecular contacts. Hirshfeld analysis has shown that S⋯H (9.2–19.8%) and O⋯H (5.4–18.2%) intermolecular hydrogen bonds as well as weak H⋯H contacts (40.2–64.0%) are predominant in all the compounds to form crystal packing.


 Please wait while we load your content...
Please wait while we load your content...