Two novel nickel(ii) and cobalt(ii) metal–organic frameworks based on a rigid aromatic multicarboxylate ligand: syntheses, structural characterization and magnetic properties†
Abstract
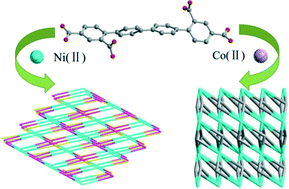
Two novel metal–organic frameworks, formulated as [Ni3(qptc)2(H2O)3]·3.5H2O·3Me2NH (1) and [Co3(qptc)2(H2O)4]·5H2O (2) (H4qptc = 1,1′:4′,1′′:4′′,1′′′-quaterphenyl-2,4,2′′′,4′′′-tetracarboxylic acid), have been hydrothermally constructed based on a rigid aromatic multicarboxylate ligand. The single-crystal X-ray diffraction analysis shows that complex 1 crystallizes in the triclinic crystal system with space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) and possesses a 4,8-connected 3D open framework including trinuclear nickel Ni3 SBUs, which belongs to an scu topology with the point symbols (416·612) and (44·62). Complex 2 belongs to the monoclinic crystal system with space group C2/c and exhibits a 3D framework containing Co3 SBUs, which is a flu topology with the point symbols (412·612·84) and (46). Furthermore, the variable-temperature magnetic susceptibilities of complexes 1 and 2 have been investigated. Both complexes show weak ferromagnetic coupling between metal ions estimated by using a linear trinuclear model for 1 in the range of 2–300 K and the PHI program for 2 in the range of 20–300 K taking the orbital contribution into consideration.
and possesses a 4,8-connected 3D open framework including trinuclear nickel Ni3 SBUs, which belongs to an scu topology with the point symbols (416·612) and (44·62). Complex 2 belongs to the monoclinic crystal system with space group C2/c and exhibits a 3D framework containing Co3 SBUs, which is a flu topology with the point symbols (412·612·84) and (46). Furthermore, the variable-temperature magnetic susceptibilities of complexes 1 and 2 have been investigated. Both complexes show weak ferromagnetic coupling between metal ions estimated by using a linear trinuclear model for 1 in the range of 2–300 K and the PHI program for 2 in the range of 20–300 K taking the orbital contribution into consideration.

 Please wait while we load your content...
Please wait while we load your content...