From isomorphous to “anisomorphous” ionic co-crystals of barbituric acid upon dehydration and return†
Abstract
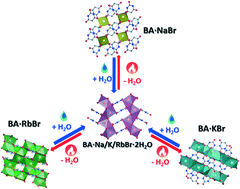
The isomorphous hydrated ionic co-crystals (ICCs) of barbituric acid (BA) of the formula BA·MBr·2H2O (M = Na, K, Rb) can be easily dehydrated to the corresponding anhydrous ICCs BA·MBr (M = Na, K, Rb). The three anhydrous crystals possess different structures that revert to the isomorphous crystals upon hydration. Only BA·RbBr is stable in air, making it possible to determine its crystal structure from single-crystal X-ray diffraction. BA·NaBr and BA·KBr rapidly absorb water from air and convert to the hydrated form. The crystal structures of BA·NaBr and BA·KBr have been determined by X-ray powder diffraction in sealed capillaries. Solid-state NMR spectroscopy was fundamental in determining the number of independent molecules in the asymmetric unit for the structural determination of the ICC BA·NaBr. The dehydration process was characterized by variable temperature X-ray powder diffraction, DSC and TGA.


 Please wait while we load your content...
Please wait while we load your content...