Self-assembly synthesis, structural features, reversible temperature-induced dehydration/hydration, and magnetic and luminescence properties of lanthanide(iii) compounds derived from 5-fluoronicotinic acid†
Abstract
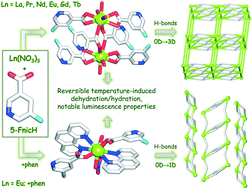
A new series of eight lanthanide coordination compounds, namely [Ln(5-Fnic)3(H2O)3]2·2H2O (Ln = La (1), Pr (2), Nd (3), Eu (4), Gd (5), and Tb (6)), [Tm(5-Fnic)2(H2O)4]2[5-Fnic]2·2H2O (7), and [Eu(5-Fnic)2(phen)2(NO3)(H2O)]·2H2O (8) were generated by a hydrothermal self-assembly method in H2O from the corresponding lanthanide metal(III) nitrates, with 5-fluoronicotinic acid (5-FnicH) as the main building block, and an optional (for 8) 1,10-phenanthroline (phen) as an ancillary ligand. All the products (1–8) were fully characterized by IR spectroscopy, elemental analysis, thermogravimetric analysis, and powder and single-crystal X-ray diffraction analyses. Compounds 1–6 are isostructural and reveal neutral dilanthanide(III) molecular units, 7 is ionic and has a cationic Tm2 core, whereas 8 displays a mononuclear structure. Distinct crystal packing patterns driven by strong intermolecular hydrogen bonds were observed in 1–6, 7, and 8, resulting in the 0D → 3D (1–7) or 0D → 1D (8) extension of the structures into H-bonded networks; their topological analysis and classification were performed. Products 1 and 3–8 constitute the first La, Nd, Eu, Gd, Tb, and Tm coordination compounds derived from 5-fluoronicotinic acid. The magnetic properties of compounds 2, 3, 5, and 7 were investigated. Besides, products 4, 6, and 8 exhibit a reversible temperature-induced dehydration/hydration behavior, which also influences their solid state luminescence properties that were studied in detail. The intensity of the emission bands of the dehydrated samples greatly increases after thermal removal of water molecules and then returns back to the original level upon exposure to water. This reversible behavior is more pronounced in 8 given the additional presence of phenanthroline moieties.

 Please wait while we load your content...
Please wait while we load your content...