Two-step synthesis of heterometallic coordination polymers using a polyazamacrocyclic linker†
Abstract
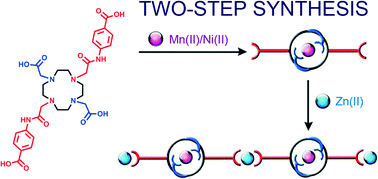
A new macrocyclic linker 1,4,7,10-tetraazacyclododecane-1,7-bis(4-acetamidobenzoic)-4,10-diacetic acid (H4L1) was synthesized and characterized. This linker was used to create two heterometallic coordination polymers following a two-step synthesis. This synthesis consisted of first combining this polyazamacrocyclic linker with Ni(II) or Mn(II) ions to obtain the corresponding metallomacrocyclic complexes showing non-coordinated carboxylic groups. In a second step, these metallated macrocycles were used as building units to construct two heterometallic Ni(II)–Zn(II) and Mn(II)–Zn(II) coordination polymers when combined with Zn(II) ions. In addition, a third Zn(II)–Zn(II) coordination polymer could also be synthesized by direct mixing of H4L1 with Zn(II) ions. Interestingly, the Mn(II)–Zn(II) coordination polymer exhibits a reversible type-I “crystal-to-amorphous transformation” upon water sorption/desorption.
- This article is part of the themed collection: 2016 New talent

 Please wait while we load your content...
Please wait while we load your content...