Strong luminescent copper(i) halide coordination polymers and dinuclear complexes with thioacetamide and N,N′-donor ligands†
Abstract
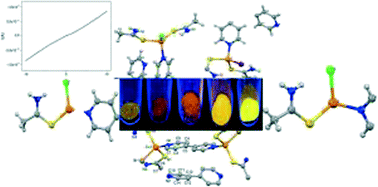
Direct reactions between copper(I) halides, thioacetamide (TAA) and 4,4′-bipyridine (4,4′-bpy) or pyrazine (pyz) in MeCN led to the formation of mixed ligand compounds [Cu2Cl2(TAA)2(4,4′-bpy)]n (1), [Cu2X2(TAA)4(4,4′-bpy)]·(4,4′-bpy) (X = Cl (2), Br (3) and I (4)) and [Cu2Cl2(TAA)2(pyz)]n (5), and the binuclear complex [Cu2I2(TAA)4] (6). The formation of these compounds strongly depends on both the ratio of the reactants and the halide. The structures of these compounds have been determined by X-ray diffraction showing interesting intermolecular interactions based on hydrogen bonding. The physical properties of these compounds show that 1–5 are strongly luminescent with red emission (500–750 nm) at room temperature in the solid state, while compound 1 behaves as a semiconductor and 5 as an insulator.

 Please wait while we load your content...
Please wait while we load your content...