Comparison of amorphous, pseudohexagonal and orthorhombic Nb2O5 for high-rate lithium ion insertion†
Abstract
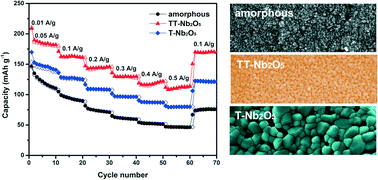
Amorphous, pseudohexagonal and orthorhombic Nb2O5 nanoparticles were synthesized using a facile and green sol–gel process followed by thermal treatment at 450 °C, 600 °C and 800 °C for 3 h in air, respectively. The resulting materials have been subjected to a detailed experimental study and comparison of their structural, electrical and electrochemical properties. The experiments have demonstrated that the pseudohexagonal Nb2O5 (TT-Nb2O5) exhibited higher storage capacity, largely due to its high specific surface area and small crystallites, and better cycling performance than both amorphous Nb2O5 (a-Nb2O5) and orthorhombic Nb2O5 (T-Nb2O5); such experimental findings were found to be associated with and thus ascribed to the lower charge transfer resistance and higher lithium ion diffusion coefficient of TT-Nb2O5 than those of a-Nb2O5 and T-Nb2O5. This research contributes to a better fundamental understanding of the relationship between the crystal structure and the crystallinity and electrochemical properties, particularly Li-ion storage properties, and leads to a possible new advancement in the research field of lithium ion batteries and pseudocapacitors.

 Please wait while we load your content...
Please wait while we load your content...