A series of Zn(ii) and Cd(ii) coordination compounds based on 4-(4H-1,2,4-triazol-4-yl)benzoic acid: synthesis, structure and photoluminescence properties†
Abstract
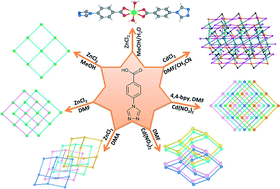
Seven coordination compounds, namely [Zn(4-tba)2(H2O)2] (1), [Zn(4-tba)Cl]·CH3OH (2), [Zn(4-tba)2] (3), [Zn(4-tba)2]·DMA·3.5H2O (4), [Cd(4-tba)2]·DMF·3.6H2O (5), [Cd(4-tba)2] (6) and [Cd5Cl4(4-tba)6]·1.5DMF·4H2O (7) (4-Htba = 4-(4H-1,2,4-triazol-4-yl)benzoic acid, DMA = N,N-dimethylacetamide and DMF = N,N-dimethylformamide), have been synthesized under hydro/solvothermal conditions by using a triazolate–carboxylate bifunctional organic ligand, 4-Htba. Complexes 1 and 2 exhibit a mononuclear motif and a two-dimensional (2D) network with sql topology, respectively, whereas 3–6 display different interpenetrating structures. Compound 3 shows a 2-fold interpenetrating 2D net with sql topology, 4 and 5 display uninodal 4-connected 4-fold interpenetrating 3D nets with dia (66) topology belonging to class Ia and class IIIa, respectively, and compound 6 exhibits a 5-fold interpenetrating dia net. Complex 7 exhibits a non-interpenetrating 3D framework constructed from decanuclear cadmium–chloride chain units and 4-tba ligands with various coordination modes. All the complexes were characterized using single-crystal X-ray diffraction, powder X-ray diffraction (PXRD), IR spectroscopy and elemental analyses. In addition, the thermogravimetric analysis and solid-state photoluminescence results for 1–7 were also investigated.

 Please wait while we load your content...
Please wait while we load your content...