Catalytic enantioselective epoxidation of nitroalkenes†
Abstract
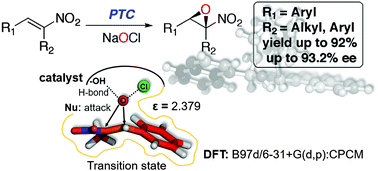
Nitroepoxides are potentially exploitable as synthons with vicinal electrophilic centers. Nevertheless, although advances have been made in the field, enantioselective epoxidation of nitroalkenes is still a challenging process. Herein we show a convenient procedure for the preparation of optically active nitroepoxides in high enantiomeric excess and high chemical yield. The kinetic data of the best catalyst have been examined using computational methods based on DFT calculations. Interestingly, the results demonstrate that the enantioselectivity of the epoxidation of nitroalkenes by this kind of catalyst is not only kinetically but also thermodynamically controlled.

 Please wait while we load your content...
Please wait while we load your content...