Long range electrostatic forces in ionic liquids
Abstract
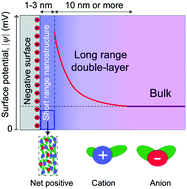
Ionic liquids are pure salts that are liquid under ambient conditions. As liquids composed solely of ions, the scientific consensus has been that ionic liquids have exceedingly high ionic strengths and thus very short Debye screening lengths. However, several recent experiments from laboratories around the world have reported data for the approach of two surfaces separated by ionic liquids which revealed remarkable long range forces that appear to be electrostatic in origin. Evidence has accumulated demonstrating long range surface forces for several different combinations of ionic liquids and electrically charged surfaces, as well as for concentrated mixtures of inorganic salts in solvent. The original interpretation of these forces, that ionic liquids could be envisioned as “dilute electrolytes,” was controversial, and the origin of long range forces in ionic liquids remains the subject of discussion. Here we seek to collate and examine the evidence for long range surface forces in ionic liquids, identify key outstanding questions, and explore possible mechanisms underlying the origin of these long range forces. Long range surface forces in ionic liquids and other highly concentrated electrolytes hold diverse implications from designing ionic liquids for energy storage applications to rationalizing electrostatic correlations in biological self-assembly.
- This article is part of the themed collections: Most cited Features from 2016, 2017, Commemorating Michael Faraday (1791-1867) and Most downloaded articles of 2017: Physical and Environmental Chemistry


 Please wait while we load your content...
Please wait while we load your content...