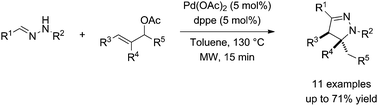
Selective Tsuji–Trost type C-allylation of hydrazones: a straightforward entry into 4,5-dihydropyrazoles†
Abstract
The 4,5-dihydropyrazole motif has drawn considerable attention over the years as it was shown to exhibit a plethora of biological and pharmacological properties, including anticancer, antibacterial, antifungal, antiviral, and anti-inflammatory properties. As such, it has been the target of a number of methods and drug discovery programs. We report here a straightforward and highly selective approach featuring a key palladium-catalysed Tsuji–Trost type C-allylation and subsequent intramolecular 1,4-addition of hydrazones.


 Please wait while we load your content...
Please wait while we load your content...