A tunable metal–polyaniline interface for efficient carbon dioxide electro-reduction to formic acid and methanol in aqueous solution†
Abstract
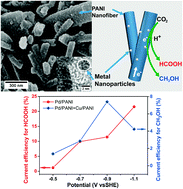
It is reported that metals on polyaniline (PANI) prepared by a simple method can exhibit excellent activity in the electro-reduction of CO2 to HCOOH or CH3OH due to tunable properties: N atoms on PANI capture CO2 through a strong Lewis acid–base interaction while Pd atoms, amongst Pd, Pt, and Cu studied, facilitate the fastest proton and electron transfers along PANI to the CO2 trapped sites to give rise to the best HCOOH yield in a highly cooperative manner.


 Please wait while we load your content...
Please wait while we load your content...