Enantioselective formation of cyclopropane spiroindenes from benzofulvenes by phase transfer catalysis†
Abstract
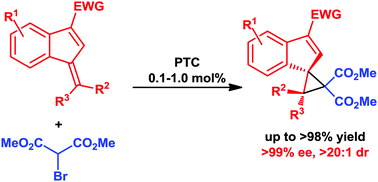
The enantio- and diastereoselective formation of indenes spirofused to highly substituted cyclopropanes is described. The application of a new cinchona alkaloid derived ammonium salt in a phase transfer catalysis setup facilitates high selectivity and excellent yields at low catalyst loadings (0.1–1.0 mol%).

 Please wait while we load your content...
Please wait while we load your content...