Construction of the carbon–chalcogen (S, Se, Te) bond at the 2,6-positions of BODIPY via Stille cross-coupling reaction†
Abstract
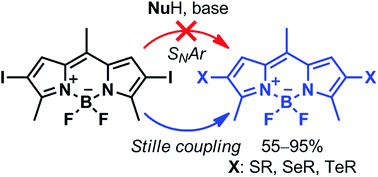
Seven new 2-chalcogen- or 2,6-dichalcogen- (S, Se, Te) BODIPY derivatives were synthesized in good to excellent yields (55–95%) by a Pd-catalyzed C–heteroatom Stille cross-coupling reaction, overcoming the limitations of SNAr. The fluorophores show interesting tunable optical properties associated with the formation of a twisted intramolecular charge transfer excited state and competing intersystem crossing.

 Please wait while we load your content...
Please wait while we load your content...