Br2F7− and Br3F10−: peculiar anions showing μ2- and μ3-bridging F-atoms†
Abstract
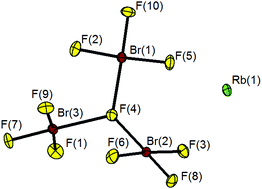
RbCl and CsCl react with BrF3 yielding the corresponding decafluoridotribromates(III), MBr3F10 (M = Rb, Cs), which were structurally characterized for the first time. The Br3F10− anion is surprisingly not linear but contains a μ3-bridging fluorine atom and seems to be the first example of μ3-F bridging of Br atoms. The compounds are highly reactive and cannot be handled in glassware. As for the tetrafluoridobromates themselves, they are powerful oxidizers and thus suitable for the dry-chemical recycling of precious metals and additionally feature a significantly higher BrF3 content.

 Please wait while we load your content...
Please wait while we load your content...