Rh-Catalyzed arylation of fluorinated ketones with arylboronic acids†
Abstract
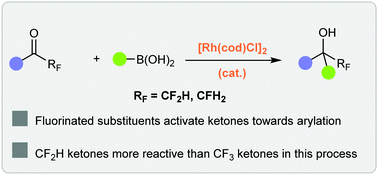
The Rh-catalyzed arylation of fluorinated ketones with boronic acids is reported. This efficient process allows access to fluorinated alcohols in high yields under mild conditions. Competition experiments suggest that difluoromethyl ketones are more reactive than trifluoromethyl ketones in this process, despite their decreased electronic activation, an effect we postulate to be steric in origin.


 Please wait while we load your content...
Please wait while we load your content...