Total synthesis of dehaloperophoramidine using a highly diastereoselective Hosomi–Sakurai reaction†
Abstract
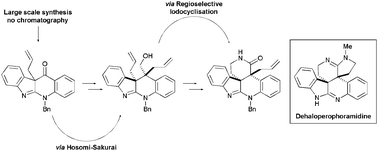
The synthesis of dehaloperophoramidine, a non-halogenated derivative of the marine natural product perophoramidine, and its biological activity towards HCT116, HT29 and LoVo colorectal carcinoma cells is reported. A [3,3]-Claisen rearrangement and an epoxide opening/allylsilylation reaction installed the contiguous all-carbon quaternary stereocentres with the required relative stereochemistry.


 Please wait while we load your content...
Please wait while we load your content...