Synthesis and application of hexagonal perovskite BaNiO3 with quadrivalent nickel under atmospheric and low-temperature conditions†
Abstract
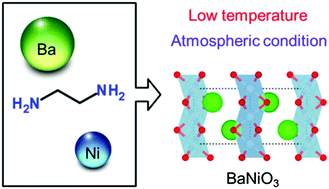
A hexagonal perovskite BaNiO3 with unusually high-valence nickel(IV) was synthesized under atmospheric and low-temperature conditions by an ethylenediamine-derived wet-chemical route. Secondary phases disappeared with increase in the pH value, and the single-phase BaNiO3 was successfully synthesized at pH 10. The specific surface area was ∼32 m2 g−1, which is significantly enhanced compared to the BaNiO3 (0.3 m2 g−1) synthesized by flux-mediated crystal growth. The BaNiO3 was used as an oxygen-evolution reaction (OER) catalyst, and the specific mass activity was ∼5 times higher than that of the BaNiO3 synthesized by flux-mediated crystal growth. As a result, the ethylenediamine-derived sol–gel synthesis could be a simple technique to prepare crystalline compounds such as perovskites and spinels, with unusually high-valence transition metals.

 Please wait while we load your content...
Please wait while we load your content...