Mechanistic study of the radical SAM-dependent amine dehydrogenation reactions†
Abstract
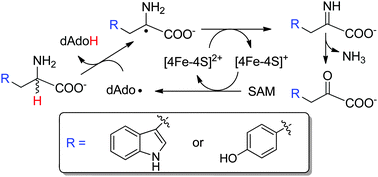
The radical SAM enzyme NosL catalyzes the conversion of L-Trp to 3-methyl-2-indolic acid, and this reaction is initiated by the 5′-deoxyadenosyl (dAdo) radical-mediated hydrogen abstraction from the L-Trp amino group. We demonstrate here that when D-Trp was used in the NosL reaction, hydrogen abstraction occurs promiscuously at both the amino group and Cα of D-Trp. These results inspired us to establish the detailed mechanism of L-Trp amine dehydrogenation catalyzed by a NosL mutant, and to engineer a novel radical SAM-dependent L-Tyr amine dehydrogenase from the thiamine biosynthesis enzyme ThiH.


 Please wait while we load your content...
Please wait while we load your content...