A mechanistic study and computational prediction of iron, cobalt and manganese cyclopentadienone complexes for hydrogenation of carbon dioxide†
Abstract
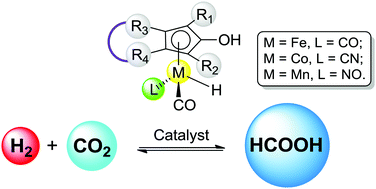
A series of cobalt and manganese cyclopentadienone complexes are proposed and examined computationally as promising catalysts for hydrogenation of CO2 to formic acid with total free energies as low as 20.0 kcal mol−1 in aqueous solution. Density functional theory study of the newly designed cobalt and manganese complexes and experimentally reported iron cyclopentadienone complexes reveals a stepwise hydride transfer mechanism with a water or a methanol molecule assisted proton transfer for the cleavage of H2 as the rate-determining step.


 Please wait while we load your content...
Please wait while we load your content...