N-Heterocyclic carbene-mediated redox condensation of alcohols†
Abstract
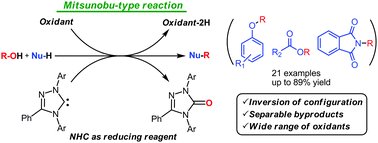
N-Heterocyclic carbenes (NHCs) with a variety of oxidants promote the Mitsunobu-type coupling reactions of alcohols with phenols, carboxylic acids, and phthalimide. Experiments using a chiral alcohol indicate that these reactions proceed via SN1 or SN2 pathways depending on the polarity of the used solvents. The NHCs are consumed as reducing reagents to form their oxides as readily separable byproducts.

 Please wait while we load your content...
Please wait while we load your content...