A decahaem cytochrome as an electron conduit in protein–enzyme redox processes†
Abstract
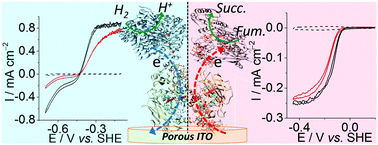
The decahaem cytochrome MtrC from Shewanella oneidensis MR-1 was employed as a protein electron conduit between a porous indium tin oxide electrode and redox enzymes. Using a hydrogenase and a fumarate reductase, MtrC was shown as a suitable and efficient diode to shuttle electrons to and from the electrode with the MtrC redox activity regulating the direction of the enzymatic reactions.


 Please wait while we load your content...
Please wait while we load your content...