13-Helix folding of a β/γ-peptide manifold designed from a “minimal-constraint” blueprint†
Abstract
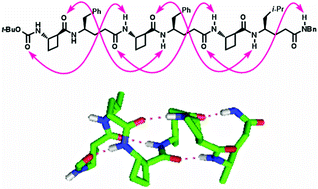
A bottom-up design rationale was adopted to devise β/γ-peptide foldamer manifolds which would adopt preferred 13-helix conformations, relying on minimal steric imposition brought by the constituent amino acid residues. In this way, a well-defined 13-helix conformer was revealed for short oligomers of trans-2-aminocyclobutanecarboxylic acid and γ4-amino acids in alternation, which gave good topological superposition upon an α-helix motif.
- This article is part of the themed collection: Foldamers

 Please wait while we load your content...
Please wait while we load your content...