Abstract
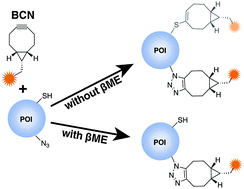
The cross-reactivity between some cyclooctynes and thiols limits the bioorthogonality of the strain-promoted azide–alkyne cycloaddition reaction. We show that a low concentration of β-mercaptoethanol significantly reduces the undesirable side reaction between bicyclononyne (BCN) and cysteine and while preserving free cysteines. We site-specifically label a genetically-encoded azido group in the visual photoreceptor rhodopsin to demonstrate the utility of the strategy.


 Please wait while we load your content...
Please wait while we load your content...