Cephalosporins inhibit human metallo β-lactamase fold DNA repair nucleases SNM1A and SNM1B/apollo†
Abstract
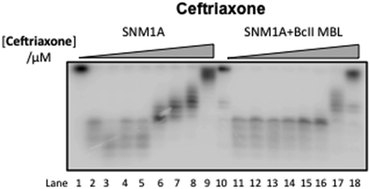
Bacterial metallo-β-lactamases (MBLs) are involved in resistance to β-lactam antibiotics including cephalosporins. Human SNM1A and SNM1B are MBL superfamily exonucleases that play a key role in the repair of DNA interstrand cross-links, which are induced by antitumour chemotherapeutics, and are therefore targets for cancer chemosensitization. We report that cephalosporins are competitive inhibitors of SNM1A and SNM1B exonuclease activity; both the intact β-lactam and their hydrolysed products are active. This discovery provides a lead for the development of potent and selective SNM1A and SNM1B inhibitors.


 Please wait while we load your content...
Please wait while we load your content...