Comparison of design strategies for α-helix backbone modification in a protein tertiary fold†
Abstract
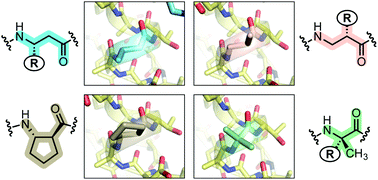
We report here the comparison of five classes of unnatural amino acid building blocks for their ability to be accommodated into an α-helix in a protein tertiary fold context. High-resolution structural characterization and analysis of folding thermodynamics yield new insights into the relationship between backbone composition and folding energetics in α-helix mimetics and suggest refined design rules for engineering the backbones of natural sequences.
- This article is part of the themed collection: Foldamers

 Please wait while we load your content...
Please wait while we load your content...