Stereoselective synthesis and structural elucidation of dicarba peptides†
Abstract
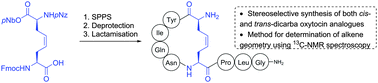
A facile stereoselective synthesis of cis and trans unsaturated dicarba peptides has been established using preformed diaminosuberic acid derivatives as bridging units. In addition, characteristic spectral differences in the 13C-NMR spectra of the cis- and trans-isomers show that the chemical shift of carbons in the Δ4,5-diaminosuberic acid residue can be used to assign stereochemistry in unsaturated dicarba peptides formed from ring closing metathesis of linear peptide sequences.

 Please wait while we load your content...
Please wait while we load your content...