Covalent triazine framework-supported palladium as a ligand-free catalyst for the selective double carbonylation of aryl iodides under ambient pressure of CO†
Abstract
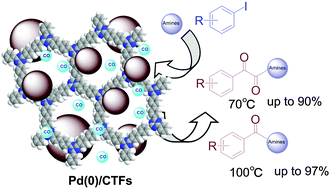
Carbonylation of aryl iodides with amines under atmospheric pressure of CO, catalyzed by Pd/CTFs (covalent triazine frameworks) without any specific additives, leads to the highly selective synthesis of α-ketoamides.

 Please wait while we load your content...
Please wait while we load your content...