Bromomethyllithium-mediated chemoselective homologation of disulfides to dithioacetals†
Abstract
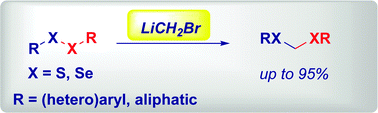
An efficient, chemoselective homologation of disulfides and diselenides to the corresponding dithio- and diselenoacetals has been developed via the addition of bromomethyllithium. Chemoselectivity is fully preserved in the presence of concomitant electrophilic sites decorating the substrates. The synthetic potential of selected dithioacetals has been evaluated in Feringa–Fañanas-Mastral-type Pd-catalyzed coupling with an organolithium and in the unusual 1,4-addition to a Weinreb amide.

 Please wait while we load your content...
Please wait while we load your content...