Efficient total syntheses and biological activities of two teixobactin analogues†
Abstract
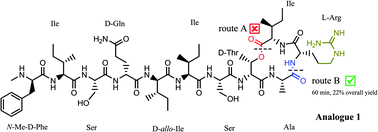
The discovery of the new antibiotic teixobactin has been timely in the race for unearthing novel antibiotics wherein the emergence of drug resistant bacteria poses a serious threat worldwide. Herein, we present the total syntheses and biological activities of two teixobactin analogues. This approach is simple, efficient and has several advantages: it uses commercially available building blocks (except AllocHN-D-Thr-OH), has a single purification step and a good recovery (22%). By using this approach we have synthesised two teixobactin analogues and established that the D-amino acids are critical for the antimicrobial activity of these analogues. With continuing high expectations from teixobactin, this work can be regarded as a stepping stone towards an in depth study of teixobactin, its analogues and the quest for synthesising similar molecules.

 Please wait while we load your content...
Please wait while we load your content...