Addition of silylated nucleophiles to α-oxoketenes†
Abstract
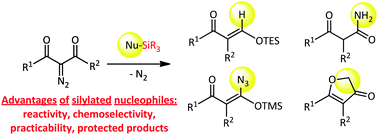
A general evaluation of silylated nucleophiles to intercept transient α-oxoketenes generated by microwave-assisted Wolff rearrangement of 2-diazo-1,3-dicarbonyl compounds is presented. Original scaffolds and synthetic intermediates are accessed in a rapid, efficient and easy-to-handle way. Mechanistic studies by DFT calculations and some post-functionalizations are discussed.

 Please wait while we load your content...
Please wait while we load your content...