Neutral Fe(iv) alkylidenes, including some that bind dinitrogen†
Abstract
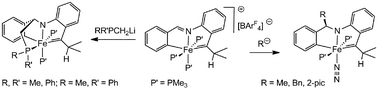
Neutral, formally Fe(IV) alkylidene species are sought as plausible olefin metathesis catalysts, and the synthesis of several is described herein. The complexes are prepared via nucleophilic attack (Nu = MeLi, PhCH2K, 2-picolyllithium, Me2PCH2Li, MePhPCH2Li, Ph2PCH2Li) at the imine of cationic [mer-{κ-C,N,C-(C6H4-yl)-2-CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N(2-C6H4-C(iPr)
N(2-C6H4-C(iPr)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) )}Fe(PMe3)3][B(3,5-CF3-C6H3)4]. In contrast, MeMgCl and mesityllithium displaced and deprotonated bound PMe3, respectively. Structural details are provided for mer-{κ-C,N,C-(C6H4-yl)-2-CH(Bn)N(2-C6H4-C(iPr)
)}Fe(PMe3)3][B(3,5-CF3-C6H3)4]. In contrast, MeMgCl and mesityllithium displaced and deprotonated bound PMe3, respectively. Structural details are provided for mer-{κ-C,N,C-(C6H4-yl)-2-CH(Bn)N(2-C6H4-C(iPr)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) )}Fe{trans-(PMe3)2}N2 and {κ-C,N,C,P-(C6H4-yl)-2-CH(CH2PMe2)N(2-C6H4-C(iPr)
)}Fe{trans-(PMe3)2}N2 and {κ-C,N,C,P-(C6H4-yl)-2-CH(CH2PMe2)N(2-C6H4-C(iPr)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) )}Fe(PMe3)2.
)}Fe(PMe3)2.

 Please wait while we load your content...
Please wait while we load your content...