An efficient approach for the construction of trifluoromethylated all-carbon quaternary stereocenters: enantioselective Ni(ii)-catalyzed Michael addition of 2-acetyl azaarene to β,β-disubstituted nitroalkenes†
Abstract
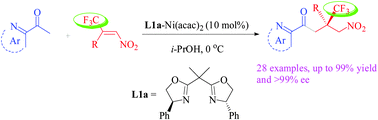
The first example of a highly enantioselective Michael addition of 2-acetyl azaarenes with β,β-disubstituted nitroalkenes was achieved using a Ni(acac)2–bisoxazoline complex as a catalyst, which afforded chiral compounds with an all-carbon quaternary stereocenter bearing a CF3 group in good yields with excellent enantioselectivities (up to >99% ee). This reaction, featuring mild conditions, excellent enantioselectivity and broad generality, provides a new efficient strategy for the construction of trifluoromethylated all-carbon quaternary stereocenters.

 Please wait while we load your content...
Please wait while we load your content...