Preparation of trypsin aptamer modified silica particles by surface initiated atom transfer radical polymerization for proteome identification†
Abstract
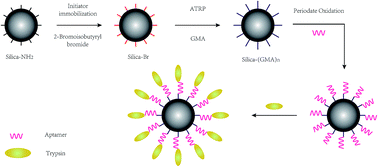
Highly efficient and complete protein digestion is vital for achieving accurate protein quantification. However, the efficiency and completeness of conventional protease free digestion are not satisfactory for extremely complicated proteomic samples. In this study, a new method of trypsin immobilization using an aptamer is developed by using surface initiated atom transfer radical polymerization (ATRP) as a linker (TAMSP-ATRP), and bovine serum albumin (BSA) was chosen as a target to investigate the enzymatic performance of ATRP modified aptamer-silica. The digestion efficiency, repeatability and recovery of the TAMSP-ATRP were evaluated by mass spectrometry (MS) analysis. Highly efficient digestion was achieved by using TAMSP-ATRP in only 2 min. Compared with traditional methods (glutaric dialdehyde as a linker and free-trypsin), the ATRP reaction as a linker obtained a BSA coverage of 62.77%, with 33 identified peptides with 0 miss cleavage, which is much better than that of glutaric dialdehyde modified trypsin digestion (sequence coverage of 33.8% and an identified 0 miss cleavage peptide number of 17) and the trypsin free digestion (coverage of 58.87% and identified 0 miss cleavage peptides of 30). In addition, human serum was digested by using TAMSP-ATRP in 2 min and trypsin free digestion in 16 h. For the TAMSP-ATRP method, 45 proteins were identified, compared to 34 proteins in trypsin free digestion, indicating that the digestion efficiency improved. All these results demonstrated that the aptamer could serve as a potential medium for the immobilization of trypsin to enhance protein digestion efficiency. Moreover, the TAMSP-ATRP was easily removed from the digestion solution. With further development, TAMSP-ATRP can be used in 18O labeling protein quantitation with the performance of suppressing the back-change of 18O labeling, which will be a huge potential advantage for online and high throughput proteome quantification.

 Please wait while we load your content...
Please wait while we load your content...