Electrothermal supercharging of proteins in native MS: effects of protein isoelectric point, buffer, and nanoESI-emitter tip size†
Abstract
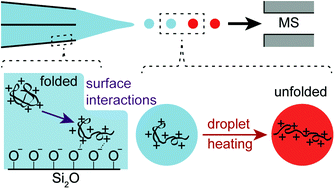
The extent of charging resulting from electrothermal supercharging for protein ions formed from various buffered aqueous solutions using nanoESI emitters with tip diameters between ∼1.5 μm and ∼310 nm is compared. Charging increases with decreasing tip size for proteins that are positively charged in solution but not for proteins that are negatively charged in solution. These results suggest that Coulombic attraction between positively charged protein molecules and the negatively charged glass surfaces in the tips of the emitters causes destabilization and even unfolding of proteins prior to nanoESI. Coulombic attraction to the negatively charged glass surfaces does not occur for negatively charged proteins and the extent of charging with electrothermal supercharging decreases with decreasing tip size. Smaller droplets are formed with smaller tips, and these droplets have shorter lifetimes for protein unfolding with electrothermal supercharging to occur prior to gaseous ion formation. Results from this study demonstrate simple principles to consider in order to optimize the extent of charging obtained with electrothermal supercharging, which should be useful for obtaining more structural information in tandem mass spectrometry.

 Please wait while we load your content...
Please wait while we load your content...