Gas-phase microsolvation of ubiquitin: investigation of crown ether complexation sites using ion mobility-mass spectrometry†
Abstract
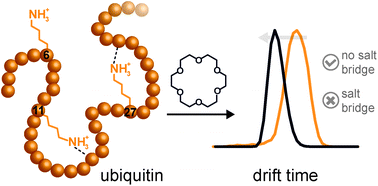
In this study the gas-phase structure of ubiquitin and its lysine-to-arginine mutants was investigated using ion mobility-mass spectrometry (IM-MS) and electron transfer dissociation-mass spectrometry (ETD-MS). Crown ether molecules were attached to positive charge sites of the proteins and the resulting non-covalent complexes were analysed. Collision induced dissociation (CID) experiments revealed relative energy differences between the wild type and the mutant crown-ether complexes. ETD-MS experiments were performed to identify the crown ether binding sites. Although not all of the binding sites could be revealed, the data confirm that the first crown ether is able to bind to the N-terminus. IM-MS experiments show a more compact structure for specific charge states of wild type ubiquitin when crown ethers are attached. However, data on ubiquitin mutants reveal that only specific lysine residues contribute to the effect of charge microsolvation. A compaction is only observed for one of the investigated mutants, in which the lysine has no proximate interaction partner. On the other hand when the lysine residues are involved in salt bridges, attachment of crown ethers has little effect on the structure.
- This article is part of the themed collection: Analyst Recent Open Access Articles


 Please wait while we load your content...
Please wait while we load your content...