Selective mass spectrometric analysis of thiols using charge-tagged disulfides†
Abstract
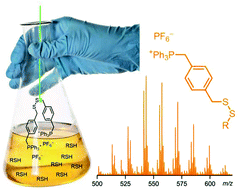
A simple chemical derivatization technique was developed for electrospray ionization mass spectrometry (ESI-MS) in which thiols and disulfides may be selectively analyzed in a complex matrix and easily characterized. These reagents enhance detection of thiols and disulfides solely due to the nature of the charge-tag derivatization agent and therefore does not require an isotopically labelled substrate. The charged disulfides readily and exclusively react with thiols in a complex matrix in a short amount of time. Furthermore, the synthesis of these reagents is simple and results in a highly pure and stable reagent. The efficacy of this reaction was demonstrated using on-line monitoring, while the scope and usefulness of the reaction was demonstrated in petroleum fractions.


 Please wait while we load your content...
Please wait while we load your content...