2-(1-Pyrenyl) benzimidazole as a ratiometric and “turn-on” fluorescent probe for iron(iii) ions in aqueous solution†
Abstract
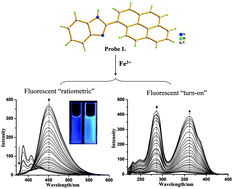
A highly selective and sensitive ratiometric and “turn-on” fluorescent probe for Fe3+, 2-(1-pyrenyl) benzimidazole (L), was synthesized by a one-step process. In emission spectra, the relative intensity ratio of excimer to monomer fluorescence (IE450/IM387) of L increased 510-fold upon the addition of 30 equiv. of Fe3+ with a detection limit of 0.2 μM (11.2 ppb) in aqueous solution. Meanwhile, the fluorescence excitation spectra of L showed a fluorescent “turn-on” probe for Fe3+ with 30-fold enhancement in excitation band intensity of excimer.

 Please wait while we load your content...
Please wait while we load your content...