Long-term non-invasive interrogation of human dorsal root ganglion neuronal cultures on an integrated microfluidic multielectrode array platform†
Abstract
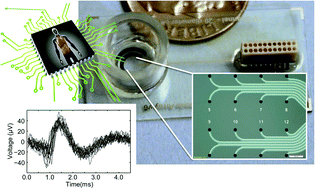
Scientific studies in drug development and toxicology rely heavily on animal models, which often inaccurately predict the true response for human exposure. This may lead to unanticipated adverse effects or misidentified risks that result in, for example, drug candidate elimination. The utilization of human cells and tissues for in vitro physiological platforms has become a growing area of interest to bridge this gap and to more accurately predict human responses to drugs and toxins. The effects of new drugs and toxins on the peripheral nervous system are often investigated with neurons isolated from dorsal root ganglia (DRG), typically with one-time measurement techniques such as patch clamping. Here, we report the use of our multi-electrode array (MEA) platform for long-term noninvasive assessment of human DRG cell health and function. In this study, we acquired simultaneous optical and electrophysiological measurements from primary human DRG neurons upon chemical stimulation repeatedly through day in vitro (DIV) 23. Distinct chemical signatures were noted for the cellular responses evoked by each chemical stimulus. Additionally, the cell viability and function of the human DRG neurons were consistent through DIV 23. To the best of our knowledge, this is the first report on long-term measurements of the cell health and function of human DRG neurons on a MEA platform. Future generations will include higher electrode numbers in customized arrangements as well as integration with different tissue types on a single device. This platform will provide a valuable testing tool for both rodent and human cells, enabling a more comprehensive risk assessment for drug candidates and toxicants.

 Please wait while we load your content...
Please wait while we load your content...