Electron-deficient acene-based liquid crystals: dialkoxydicyanopyrazinoquinoxalines†
Abstract
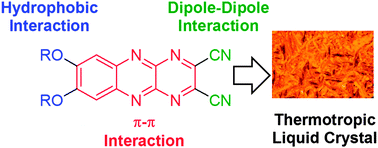
Three electron-accepting dialkoxydicyanopyrazinoquinoxaline derivatives (1a, 1b, and 1c) showed properties of smectic (Sm) liquid crystals. Temperature-dependent X-ray diffraction studies were consistent with the formation of a bilayer structure through the π-overlap and interdigitation of alkoxy chains in the Sm liquid crystalline state. Intermolecular dipole–dipole interactions between the cyano groups played an important role in stabilizing the bilayer structure and liquid crystalline properties. Elongation of the alkoxy chains from C6H13O- (1a) and/or C12H25O- (1b) to C18H37O- (1c) changed the molecular arrangements and the liquid crystal phase from SmA to SmC, suggesting the importance of the van der Waals interaction of CnH2n+1O- chains for stabilizing the liquid crystalline phase. A hole-mobility value of 5 × 10−3 cm2 V−1 s−1 was observed for the SmA phase of 1b at 438 K based on transient photocurrent measurements.

 Please wait while we load your content...
Please wait while we load your content...