Deep red emission in Eu2+-activated Sr4(PO4)2O phosphors for blue-pumped white LEDs
Abstract
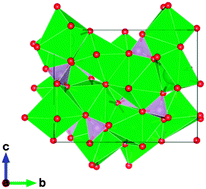
The deep red phosphor Sr4(PO4)2O:Eu2+, which has an excitation peak around 450 nm for blue LED applications, is reported. This behavior is unusual for most phosphate phosphors. The crystal structure of Sr4(PO4)2O:Eu2+ is found to be monoclinic P21 and isotypic with Ca4(PO4)2O:Eu2+, which also shows deep red emission. Sr4(PO4)2O:Eu2+ has a larger lattice volume than Ca4(PO4)2O:Eu2+, but their emission and excitation spectra at room temperature are very similar. The key factors are discussed for achieving a large redshift of the 5d levels of Eu2+ ion in order to emit red light. In particular, the importance of the anion polarizability and the distortions of the metal coordination polyhedra are discussed, including the effective coordination number. Importantly, Sr4(PO4)2O:Eu2+ lacks the yellow emission at 77 K, which is found in Ca4(PO4)2O:Eu2+. The differences in thermal quenching behavior for Eu2+ dopants in Sr4(PO4)2O:Eu2+ and Ca4(PO4)2O:Eu2+ are attributed to the degree of auto/photo-ionization due to differences in the band gaps of these compounds. The importance of the large band gap of the host lattice in avoiding non-radiative processes of energy relaxation was confirmed.
- This article is part of the themed collection: In celebration of Tony Cheetham’s 70th birthday

 Please wait while we load your content...
Please wait while we load your content...