Higher and lower supramolecular orders for the design of self-assembled heterochiral tripeptide hydrogel biomaterials†
Abstract
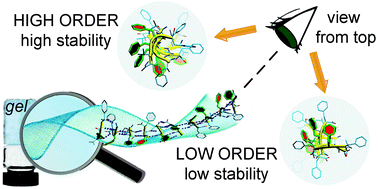
The self-assembly behaviour of the eight stereoisomers of Val–Phe–Phe tripeptides under physiological conditions is assessed by several spectroscopy and microscopy techniques. We report the first examples of self-organised hydrogels from tripeptides in the L–D–L or D–L–D configuration, besides the expected gels with the D–L–L or L–D–D configuration, thus widening the scope for using amino acid chirality as a tool to drive self-assembly. Importantly, the positions of D- and L-amino acids in the gelling tripeptides determine a higher or lower supramolecular order, which translates into macroscopic gels with different rheological properties and thermal behaviours. The more durable hydrogels perform well in cytotoxicity assays, and also as peptides in solution. An appropriate design of the chirality of self-assembling sequences thus allows for the fine-tuning of the properties of the gel biomaterials. In conclusion, this study adds key details of supramolecular organization that will assist in the ex novo design of assembling chiral small molecules for their use as biomaterials.

 Please wait while we load your content...
Please wait while we load your content...