Maleimide-based acyclic enediyne for efficient DNA-cleavage and tumor cell suppression†
Abstract
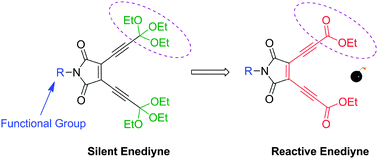
A pH-sensitive acyclic enediyne (1) was synthesized for efficient DNA-cleavage and tumor cell suppression. Unlike other acyclic enediynes, this novel enediyne transforms into a highly reactive enediyne (2) in an acidic environment only, which undergoes Bergman cyclization spontaneously at ambient temperature. An EPR study on the enediyne 2 confirmed the generation of free radicals through Bergman cyclization. The activated enediyne induced DNA-cleavage and exhibited cytotoxicity towards various tumor cells under the action of diradicals arising from spontaneous Bergman cyclization at physiological temperature. These findings suggest a novel strategy of anticancer drug design, where the activation of the silent compound takes place under the acidic environment inside the tumor cells.
- This article is part of the themed collection: Porous Materials (FEZA 2014)


 Please wait while we load your content...
Please wait while we load your content...