Structure and solubility behaviour of zinc containing phosphate glasses†
Abstract
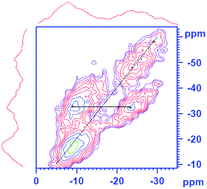
The structure of phosphate glasses of general composition 10Na2O : (20 + x/2)ZnO : (20 + x/2)CaO : (50 − x)P2O5 (0 ≤ x ≤ 20) has been investigated using IR spectroscopy, 1D 31P and 43Ca MAS Bloch decay, 31P–31P double quantum MAS-NMR and 43Ca and 67Zn static NMR techniques, as well as neutron diffraction analysis. Zinc is shown to aid glass formation in this system. Glass transition temperature and density increase with increasing cation : phosphate ratio. However, free volume calculations show structures becoming significantly more compact from x = 5 to x = 10. The structural data confirm depolymerisation of the glasses with increasing cation : phosphate ratio. Zinc oxide is found to act in a network forming role in the system, with 67Zn NMR and neutron diffraction analysis confirming zinc exhibits predominantly four-coordinate geometry. Solubility in deionised water and tris/HCl buffer solution is seen to decrease significantly with increasing x-value. This is discussed in terms of water ingress and the degree of structural openness, associated with increased cross-linking and a decrease in concentration of P–O–P linkages. pH measurements confirm invert phosphate compositions maintain physiological pH levels on immersion in water and buffer solutions for up to four weeks.

 Please wait while we load your content...
Please wait while we load your content...