VO2/rGO nanorods as a potential anode for sodium- and lithium-ion batteries†
Abstract
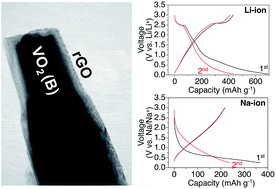
VO2 (B) is an interesting cathode candidate in lithium-ion batteries with a high theoretical capacity and fast charge/mass transfer rate. Most of the studies on this material have focused on the lithium insertion reaction in the range of 4.0–1.0 V. In this paper, VO2/rGO nanocomposite was prepared by a microwave-assisted solvothermal method and investigated in both lithium-ion and sodium-ion batteries as a potential anode. The VO2 (B) nanorods have an average diameter of ∼200 nm, and is encapsulated by reduced graphene oxide (rGO) nanosheets. Electrochemical results reveal that the VO2/rGO electrodes exhibit stable cycling and good rate performance in both Li and Na cells. Reversible capacities of 400 mA h g−1 over 400 cycles (vs. Li/Li+) and 200 mA h g−1 over 200 cycles (vs. Na/Na+) have been achieved, indicating it is potential as an anode candidate either in lithium- or sodium-ion batteries. Nevertheless, the reaction mechanisms seem to be different in Li and Na cells. The crystal structure of VO2 (B) is maintained at a low discharge potential of 0.05 V in lithium-ion cells, while phase amorphization occurs in sodium-ion cells below 0.5 V. This result is consistent with the previous study on TiO2, further confirming that some stable metal oxides may show rather different behaviors in Na cells than expected.

 Please wait while we load your content...
Please wait while we load your content...